Beginner’s guide to the production of AAV vectors for gene therapy – Mentored by a CDMO
Discussion with Scientist Pekka Puolasmaa and Project Manager Tuomas Nikula.

The goal of gene therapy is to treat or cure severe diseases by carrying genetic material to the target cells to repair the incorrect or missing function. Over the next several years we are likely to see these approaches become mainstream, providing hope and help to many patients with the most severe disorders such as rare diseases and cancer [1, 2]. Treatments based on Adeno-Associated Virus (AAV) vectors have already shown potential in the cure of such diseases as a rare type of vision loss, haemophilia, and spinal muscular atrophy [3, 4, 5]. Therefore, it is not surprising that as many as 44% of a total of 1370 unique gene therapy products under development are utilizing AAV [6]. The development time for viral vector-based therapies is also shorter compared to conventional drug development. FDA’s fast track designations have recently been given to AAV- based gene therapies for Fabry disease, Duchenne Muscular Dystrophy, and age-related eye disease, AMD [7,8,9].
Nevertheless, the journey to the clinical use of AAV-based new gene therapies is not easy. A thorough understanding of both AAV production and related analytics is a must when a timely entry to first-in-human studies is desired. So, what are the crucial elements of AAV production and analytics that you should be aware of when taking your AAV gene therapy design from the laboratory bench to the clinic? We at Biovian have collected a beginner’s guide to AAV vector production, which explains how the process is carried out using the platform approach at our EMA certified and FDA inspected facility. Here, Scientist Pekka Puolasmaa and Project Manager Tuomas Nikula will give answers to seven important questions:
- What are the advantages and limitations of AAV vectors?
- What plasmids are needed to produce AAV vectors?
- How are AAV vectors produced?
- How are AAV vectors purified?
- What Quality Control assays are needed for AAV vectors?
- What are the requirements for AAV vector Fill and Finish?
- How long does it take to produce AAV vectors?
1. What are the advantages and limitations of AAV vectors?
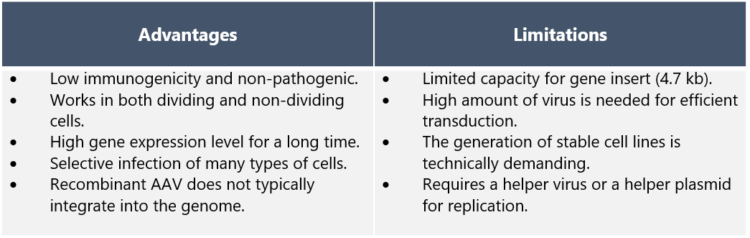
Why are AAV vectors considered so attractive for gene therapy? “There are many reasons for this,” Dr. Nikula explains. “The safety profile of AAV is considered high as wild-type AAV is not known to cause disease, and genes are removed from the recombinant AAV vectors in order to prevent replication. AAVs are also low in immunogenicity, which means they only cause mild immune response – a desired feature as AAV vectors are administered directly to the patient.
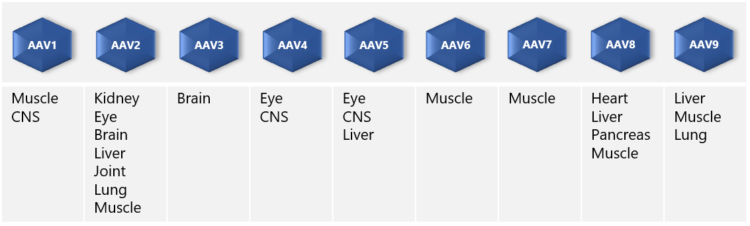
“AAVs have the ability to infect many tissue types such as muscle, lung, heart, eye, and the central nervous system. In addition, infection is not dependent on cell division. Long-term gene expression of AAVs is of great advantage in gene therapy applications.” ”On the downside,” Dr. Nikula points out, “one of the limitations of AAV vectors that should be noted in drug development is the limitation in the size of the inserted therapeutic gene. The maximum size is ~5 kb in length – although a dual AAV system may be used to enable the delivery of larger genes. Also, the production of stable AAV producer cell lines needs several steps and good planning,” he adds.
2. What plasmids are needed to produce AAV vectors?
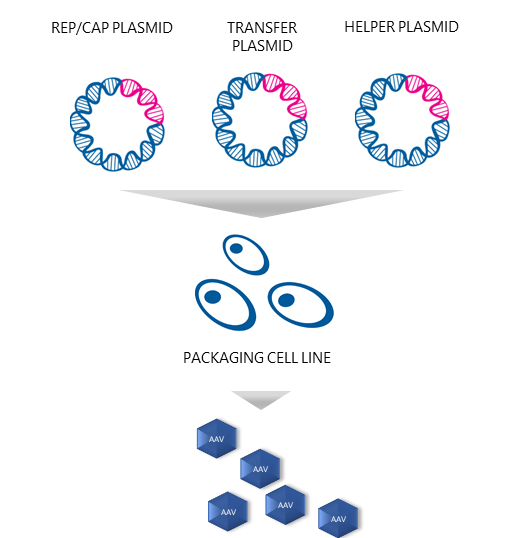
Plasmids are the building blocks needed to produce AAV vectors. “The most common approach to AAV production is the triple transfection strategy, also called transient transfection,” Dr. Nikula says. So, what plasmids are needed to produce functional recombinant AAVs with this technique? “As the name of the technique implies, three types of plasmids are needed: 1) a plasmid that carries the therapeutic gene, 2) a plasmid that contains the protein-coding genes called rep and cap, which are needed for AAV replication and capsid formation, and 3) a plasmid that enables AAV replication in the host cells,” he replies.
Why is access to quality plasmids important?
Currently, access to quality plasmids is a recognized bottleneck in AAV production[12]. “Therefore,” according to Dr. Nikula, “it is important to make sure that the availability of plasmids will not slow down your AAV project. When the target is a timely transition from pre-clinical and clinical phases to commercial manufacturing, it is essential that GMP requirements are adhered to from an early stage. GMP encompasses the entire chain of manufacturing, starting from raw materials, including plasmids. Thus, teaming up with a CDMO that can provide GMP-grade production of both plasmids and AAVs under the same roof can save you the work and effort with plasmid sourcing and quality requirements.”
How are AAV plasmids produced?
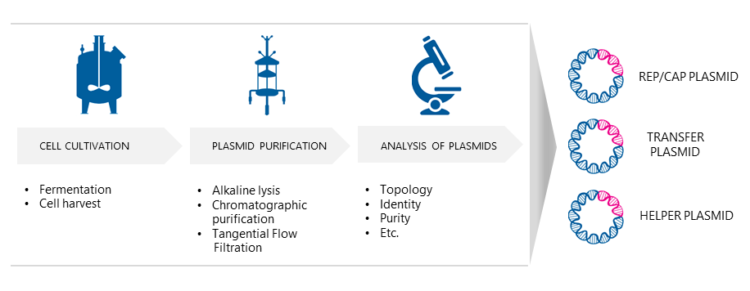
Three separate E. coli processes are needed to produce the necessary AAV plasmids for transfection. Dr. Nikula explains that first, the bacterial cells are cultured under optimized growth conditions. “Biovian uses fed-batch fermentation as one option, where nutrients are fed to the fermentor during cultivation either continuously or intermittently. The microbial cells are then harvested using centrifugation. To release the intracellular plasmids the cultured host cells are disrupted using alkaline lysis. Then the AAV plasmids are purified with chromatographic methods. Finally, the plasmids will be analyzed e.g. for their identity and purity, and E.coli master and working cell banks are generated.”
What quality control assays are deeded for AAV plasmids?
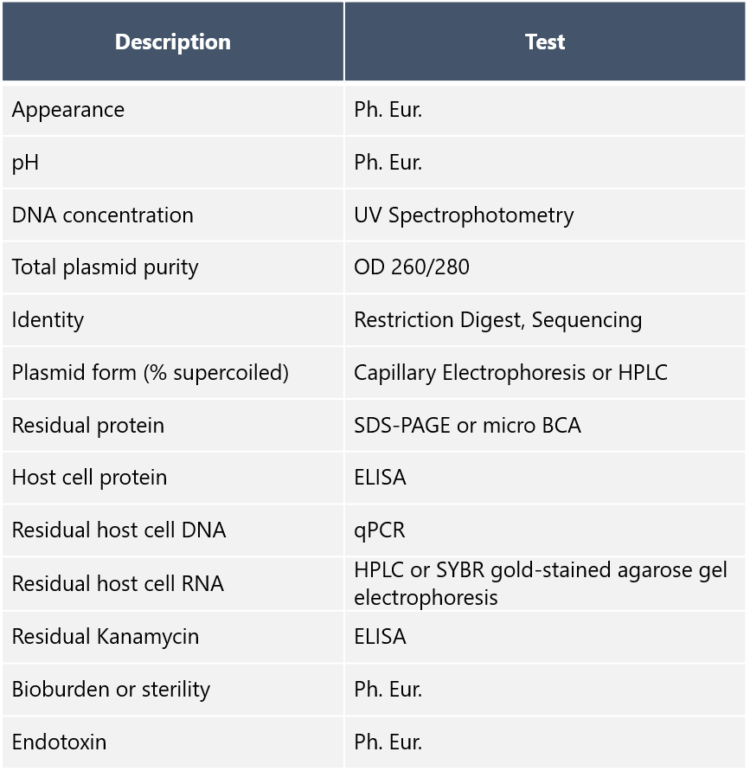
AAV plasmids can occur in compact supercoiled form, open circular form, or linear form. These so-called topological isomers may have different transfection efficiency. Dr. Nikula says that this is the reason why topology analysis with capillary electrophoresis or HPLC is among the important analyses to perform. So which form is best suited for transfection? “The supercoiled form,” he replies. “This is because supercoiled plasmids reach the perinuclear region of mammalian host cells, from where they become entrapped in the nuclei during cell division. Because of that, an AAV plasmid production protocol that consistently produces a high percentage of supercoiling is valuable. It goes without saying that the identity of the plasmids must be confirmed – at our site we use restriction enzyme mapping. Other standard QC tests performed by us at Biovian include total plasmid purity with agarose gel electrophoresis, endotoxin assay, and analyses for residual host cell RNA and DNA.” Dr. Nikula tells that impurities must carefully be eliminated from the plasmid preparations, because the identity, purity, and safety of AAV vectors are essential, and have to be demonstrated throughout the process. Examples of the AAV plasmid DNA quality control assays are listed in the table below:
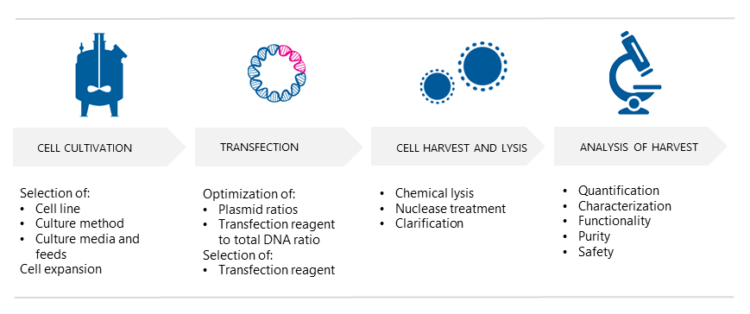
3. How are AAV vectors produced?
AAV vectors are produced in mammalian producer cell lines, typically HEK293 or HEK293T, using either adherent or suspension culture. So, which culture method should one choose? Scientist Pekka Puolasmaa replies that the benefit of suspension cultures is that they can more easily be scaled up because cell growth is limited by the concentration of cells in the medium and not by the surface area, which is the case with adherent cell cultures. “Moreover, if needed, suspension cells can be cultured in serum-free media and are thus free from bovine-derived impurities such as TSE and BSE. “On the other hand,” he continues “suspension cell cultures require daily cell counts and viability determination for growth monitoring, whereas adherent cell cultures can be inspected visually under the microscope. Also, there are AAV constructs that do not produce good yields in suspension cultures. We at Biovian provide assistance with the selection of the producer cell line as well as culture type, based on the AAV serotype and other factors and needs.”
What happens in triple transfection?
Prior to transfection, the producer cells are cultured and expanded. Puolasmaa says that the successful production of AAV vectors using triple transfection includes determining the optimal ratio of the three plasmids, and the ratio of the transfection reagent to the total amount of DNA. “AAV belongs to the genus Dependoparvovirus and normally depends upon a helper virus such as adenovirus for replication.” But would not co-infection with adenovirus contaminate the AAV stock and limit its potential use? “Yes, it would,” Puolasmaa replies. “Therefore, the necessary adenovirus genes are provided in the helper plasmid format. When helper plasmids transfect HEK293 cells, together with the two other plasmids, i.e. the transfer plasmids and the rep/cap plasmids, the AAV vectors are produced as efficiently as when helper adenovirus is used. It also eliminates the need for heat inactivation, as there are no contaminating helper adenoviruses, and thus improves the yield of recombinant AAVs.”
How are AAVs harvested and tested?
Intracellular AAVs are released into the culture media by disrupting the cultured mammalian host cells using chemical lysis. “The composition of the lysis buffer depends on the type of culture,” Puolasmaa tells. “Nuclease enzymes such as Benzonase can be added during or after cell lysis in order to digest residual nucleic acids of the producer cells and plasmids. The subsequent clarification process involves the removal of cellular debris from the viral supernatant and is done using depth filtration.” What are the essential analyses needed to analyze the harvest? “At Biovian the harvest is analyzed with qPCR or ddPCR for accurate quantification and characterization of AAV vectors,” Puolasmaa replies.
4. How are AAV vectors purified?
The effective purification of AAV vectors is an extremely important step in order to remove contaminants that originate from host cells and the culture medium. At the same time, the AAV product will be concentrated. Puolasmaa tells that Biovian’s multi-step platform purification process is compatible with the multiple AAV serotypes. “This optimized method generates AAV preparations of high purity, enriched for capsids with full vector genomes while minimizing the number of empty capsids.”
What do these purification steps involve? “The purification process starts with affinity chromatography where the resin and column size will be selected based on the virus load. AAV capsids effectively bind to antibody fragments on the affinity chromatography resin“, Puolasmaa explains. “However, the affinity resin also binds empty capsids, which lack the vector genome and do not provide a therapeutic benefit. Empty capsids are unwanted also because they may increase the immune responses in clinical use. Therefore anion exchange chromatography, AIEX, is used as the next step in AAV purification. Empty capsids are removed based on the difference in charge between the two populations. Anion exchange chromatography also effectively removes host cell proteins and host cell DNA contaminants. An additional polishing step using cation exchange chromatography, CIEX, can be added for further purification if required.”
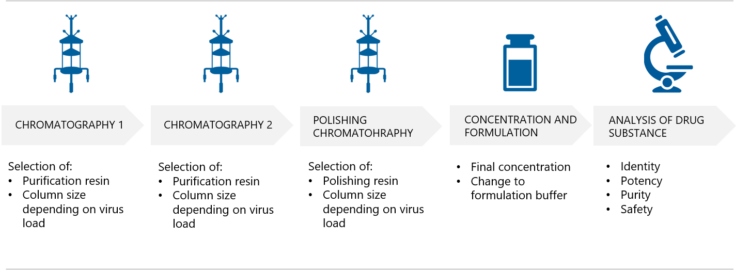
How is the final AAV concentration and formulation performed?
The final concentration of the AAV product and the exchange to formulation buffer is performed using tangential flow filtration, TFF. Puolasmaa explains that the goal of formulation optimization is to enhance the chemical and physical stability and thus prevent degradation of the AAV gene delivery system. “Optimized formulation may also enhance the transduction efficiency and thereby the effectiveness of the AAV vector in vivo.”
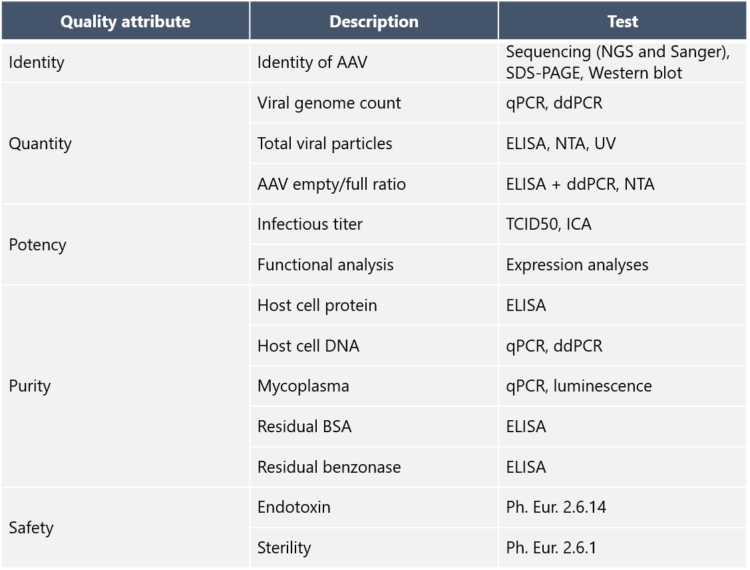
5. What Quality Control assays are needed for AAV vectors?
Purification intermediates are analyzed to confirm the presence and identity of AAV vectors and to measure viral titers and purity. How has Biovian as a CDMO addressed this need? “Biovian provides clients with full access to process analytics and documentation packages that demonstrate the quality attributes mandatory for GMP Viral Vector batches,” Puolasmaa says. An overview of the suggested quality control methods and the documentation package is shown in the tables below:
Overview of the AAV vector QA documentation package
- TSE/BSE certificate
- Certificate of Analysis
- Certificate of GMP compliance
- Comprehensive production summary report
6. What are the requirements for AAV vector Fill and Finish?
The manufacture of the Final Drug Product involves filling AAVs into vials, visual inspection, labeling, and packaging. Fill and Finish of AAV vectors are performed in dedicated rooms that fulfill BSL 2 requirements. BSL 2 facilities include enhanced safety measures, and access to the facility is restricted and monitored. Personnel working at the BSL 2 filling line are expected to take extra care with safety precautions and cleanliness. Does Biovian also provide Fill and Finish of AAVs for its clients? “Yes we do – it is one of our core competencies,” Puolasmaa answers. “Biovian’s automated aseptic filling line for AAVs is validated for up to 1000 vials per batch. The finished AAV vials may either be kept in intermediate storage at Biovian or be directly shipped to the clinic, once the batch has been released by one of our qualified persons.”
7. How long does it take to produce AAV vectors?
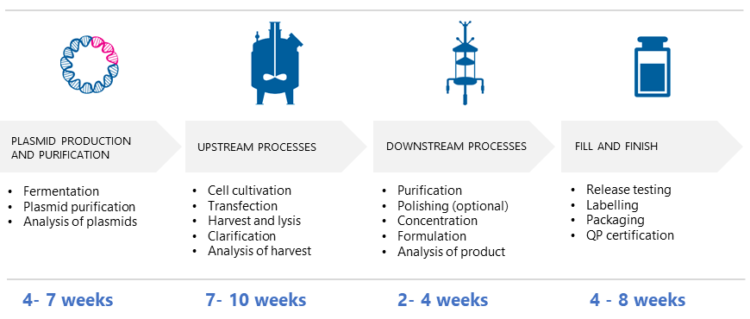
According to Puolasmaa, the time needed for AAV vector production depends on various factors, such as the selected cell line, the cultivation, and bioreactor type, the optimized lysis method, and the purification process and upscaling. “Some steps in upstream and downstream process development can be performed in parallel to speed up the process. While there is no one-size-fits-all solution, an estimated time frame from GMP plasmid production to formulation and QC-testing is illustrated in the picture below.”
Summary
AAV vectors are considered attractive vehicles for gene therapy because of their safety profile and effective gene delivery to a broad range of dividing and non-dividing cells. The production of AAV vectors requires access to quality plasmids of three types for triple transfection. In the above Project Manager, Tuomas Nikula, and Scientist, Pekka Puolasmaa have described how Biovian’s platform approach to AAV production addresses many of the important steps in AAV manufacturing. They emphasize that any platform approach should be flexible enough to accommodate several types of AAV vectors. The optimized AAV production platform of Biovian is designed to generate AAV preparations of high purity, enriched for capsids with full vector genomes while minimizing the number of empty capsids. Appropriate analytical methods are needed throughout the process for the demonstration of factors that are essential for product safety.
References
[1] Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics
[2] Rare Disease Information. https://rarediseases.org/for-patients-and-families/information-resources/rare-disease-information/
[3] FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-gene-therapy-treat-patients-rare-form-inherited-vision-loss#:~:text=FDA%20News%20Release-,FDA%20approves%20novel%20gene%20therapy%20to%20treat%20patients%20with,form%20of%20inherited%20vision%20loss&text=The%20U.S.%20Food%20and%20Drug,that%20may%20result%20in%20blindness.
[4] Gene Therapy for Patients with Hemophilia A. https://www.jwatch.org/na50591/2020/01/01/gene-therapy-patients-with-hemophilia
[5] FDA Approves Zolgensma, Landmark AAV-Delivered Gene Therapy to Treat Spinal Muscular Atrophy. https://www.asgct.org/research/news/may-2019/fda-approves-zolgensma-gene-therapy#:~:text=Gene%20Therapy%20Approval-,FDA%20Approves%20Zolgensma%2C%20Landmark%20AAV%2DDelivered%20Gene%20Therapy,to%20Treat%20Spinal%20Muscular%20Atrophy&text=Zolgensma%20(Novartis%2C%20AveXis)%2C,Food%20and%20Drug%20Administration%20today.
[6] Source, Global Data (accessed September 2020).
[7] 4D Molecular Therapeutics Receives FDA Fast Track Designation for 4D-310 Gene Therapy for Treatment of Fabry Disease. https://www.businesswire.com/news/home/20200813005246/en/4D-Molecular%20Therapeuticss-Receives-FDA-Fast-Track
[8] Pfizer Wins FDA Fast Track for Duchenne Muscular Dystrophy Treatment. https://www.fdanews.com/articles/199343-pfizer-wins-fda-fast-track-for-duchenne-muscular-dystrophy-treatment
[9] Gyroscope Therapeutics Granted FDA Fast Track Designation for GT005, an Investigational Gene Therapy for Dry Age-Related Macular Degeneration.https://www.businesswire.com/news/home/20200921005923/en/Gyroscope-Therapeutics-Granted-FDA-Fast-Track-Designation-for-GT005-an-Investigational-Gene-Therapy-for-Dry-Age-Related-Macular-Degeneration%22
[10] Adeno-associated Virus Serotypes: Vector Toolkit for Human Gene Therapy. https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(06)00204-8?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1525001606002048%3Fshowall%3Dtrue
[11] In vivo tissue-tropism of adeno-associated viral vectors. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5138125/
[12] Gene Therapy: Can CDMOs Solve the Manufacturing Bottleneck? https://www.labiotech.eu/sponsored/manufacturing-gene-therapy-bottleneck/


