Overview
Ever since it was founded in 2003 Biovian has been dedicated to providing CDMO excellence in biopharmaceutical manufacturing. Biovian especially focuses on viral vector production (Adenovirus and AAV), microbial production of recombinant proteins, and plasmid DNA. We are experts in process development, scale-up, clinical manufacturing as well as technology transfer, and we cover both Drug Substance and Drug Product manufacturing. Guided by our Nordic ethos we have established a reputation for delivering high-quality work on time and within budget to a global client base.

Our Vision
Taking biopharmaceuticals to the next level is our vision and our reason for being. We provide services so that patients around the world can benefit from the innovations of our clients. We are proud of our role in the process that aims to bring drug candidates from the laboratory bench to the clinic. Together with our clients, we follow the molecules through clinical phases and to the market. We also take the biopharmaceuticals to the next level in terms of quality.
Our Mission
Biovian is Manufacturing Happiness to both employees and clients through a working culture that values togetherness as a driver of high performance. When skilled colleagues help each other, high performance and client satisfaction will follow. Client happiness is our happiness –Manufacturing Happiness is a cycle.
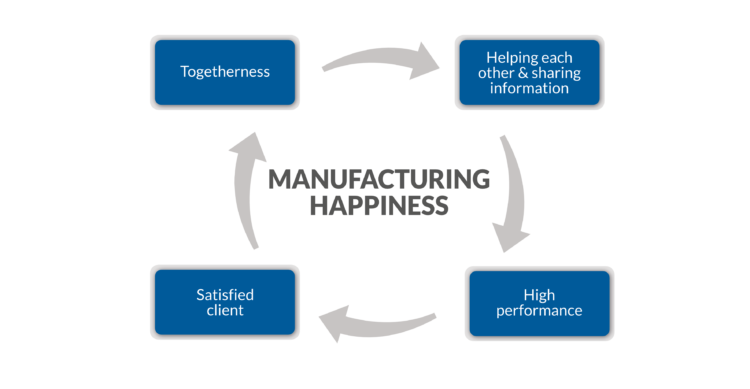
Our Values
Our values, Courage, Curiosity, and Responsibility shape the way we work. While being a global player, Biovian is still essentially a Nordic CDMO, and our approach is based on the culture of the Nordic region.
COURAGE – We dare because we care
- We care about the success of our clients and of Biovian
- We embrace transparency at all times.
- We carry responsibility together.
CURIOSITY – We keep our minds fresh to always seek the best
- We ask questions.
- We seek solutions.
- We update ourselves with new information.
- We cultivate a flexible mindset and attentiveness.
RESPONSIBILITY – We do what we say!
- We focus on actions that bring client projects to the finish line on time and within budget.
- We are committed to quality and regulations.
- We share information, we help, and we collaborate.
- We uplift each other in our daily work.
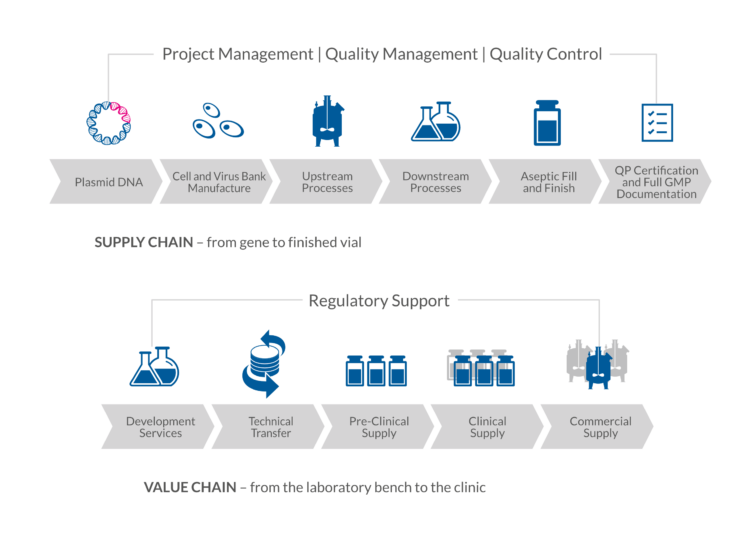
One-Stop-Shop CDMO concept
Biovian’s concept of a One-Stop-Shop is to provide clients with services through the supply chain and through the value chain. This is facilitated by the fully integrated infrastructure of resources and capabilities of Biovian. In the supply chain, Biovian’s services span from Master Cell and Virus Bank manufacturing to the Qualified Person-approved release of the final labeled drug product. Similarly, in the value chain, Biovian’s services run from the preclinical supply up to the commercial supply or manufacturing, enabling us to continue supporting clients as they take molecules through the development and onto the market. At each stage, Biovian adheres to GMP and operates out of fully inspected and fully certified facilities.
Who We Are
Our team consists of experts with Ph.D., M.Sc., or B.S. in biotechnology, biochemistry, bioengineering, virology, genetics or pharmacy, quality professionals, and supporting personnel. Having passed through Finland’s top-ranking education system, we share a focus on high quality, honesty, and reliability. The result is a CDMO that has the expertise and scientific skills needed for challenging projects and that is driven to deliver on its promises.
Whom We Serve
We offer worldwide support for biopharma drug development projects at every stage −from pre-clinical to Phase I-III and commercial supplies, depending on the scale and application. We understand the special needs of the different project steps and we serve projects of various sizes.
Our Facilities
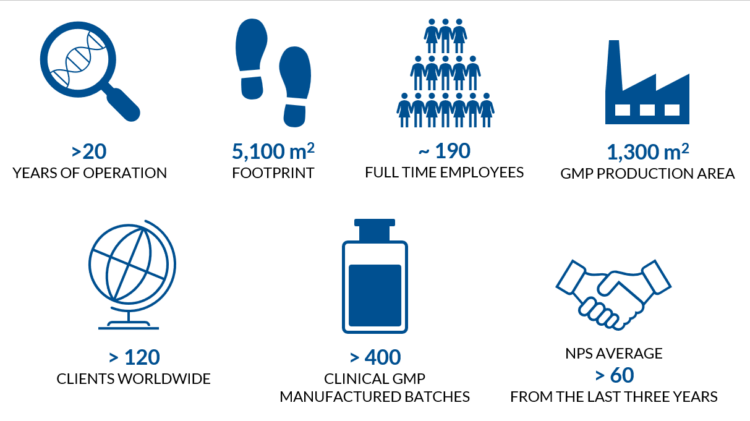
The facilities of Biovian, encompassing 5100 m2, are located in Turku Science Park in Turku, Finland, with excellent connections through both the Turku and Helsinki international airports. The facilities and processes are EMA certified and FDA inspected for GMP production of both investigational and commercial medicinal products. We are continuously expanding our production facilities to enable us to support versatile cutting-edge biopharmaceutical manufacturing needs.
Why Choose Us
Biovian offers well-established and innovative One-Stop-Shop GMP solutions. We will find a way to manage both the entire supply chain and the entire value chain to meet your needs. When partnering with us there is no need to change providers, which can be both time-consuming and expensive. You can count on our skilled and passionate workforce to find the most suitable path and to guide you through your clinical journey. We can make your CMC-journey easier.
A Word from Our CEO
“We Are more than a CDMO – We Care“
For us, at Biovian, people are always in focus. We foster and encourage an open dialogue and value a partnership with our clients. It is extremely important that our clients can feel completely safe and have trust in Biovian when it comes to keeping information confidential and providing agreed deliverables. We operate according to mutually set targets and provide our clients with the agreed products on time and within budget.
We start the partnership with a reflective, mutually transparent discussion to outline your specific needs. Whether you are a biotech startup or a larger pharma company, a well-designed strategy will keep your drug development project on a cost-efficient and regulation-compliant path during clinical development and manufacturing. Our teams are working closely with your company to reach an understanding of your objectives, requirements and challenges. This type of close collaboration we at Biovian see as truly personalized contract manufacturing.
“Our comprehensive understanding of the design of manufacturing processes helps you avoid delays and additional costs associated with last-minute changes in production technologies.”
Our highly qualified employees are committed to taking care of your products for every milestone. We are very proud of our multidisciplinary project teams, caring for the client project demands, down to the smallest detail. Biovian has its own team of qualified persons (QPs), who ensure that the client’s investigational, medicinal products are released for clinical trials or onto the market without any unexpected hurdles.
Our company is privately-owned, independent, and entirely focused on customer projects. Biovian’s strong balance sheet combined with a steady flow of new client projects as well as several returning clients makes Biovian a reliable and secure choice in the biopharma CDMO market.
“We do what we say”. This expression encapsulates the way Biovian has served a global client base for well over a decade. Our straight-forward way of approaching tasks originates from the Nordic ethos, where being as good as one’s word is a value of highest priority. We believe that personal contacts, friendliness, and reliability are essential in customer relationships. We are ready to serve our new, current, and returning clients with Nordic consistency, reliability, and efficiency.
– Antti Nieminen, CEO

