Plasmid DNA Projects and Applications
Biovian manufactures plasmid DNA for a variety of client projects. One of our main focus areas is viral vector manufacturing for those gene therapies, where plasmid DNA constructs serve as key raw material. Our GMP plasmid DNA may also be used to develop novel DNA or mRNA vaccines and non-viral gene therapy applications. We have the flexibility to deal with plasmid DNA projects of various sizes. The options for E. coli fermentation for GMP plasmid DNA production extend up to 200 L.
One-Stop-Shop CDMO for Plasmid Manufacturing
By choosing a One-Stop-Shop GMP plasmid DNA production service you get the benefit of having only one supplier – Biovian – through the entire process development and manufacturing assignment, from GMP Cell Banking to aseptic Fill and Finish. Our platform approach to plasmid DNA production minimizes the time needed from project set-up up to Drug Substance or Drug Product delivery. Quality control and documentation packages will be provided according to your product-specific requirements.
-
- Research Cell Bank (RCB)
- Master Cell Bank and Working Cell Bank (MCB/WCB)
-
- Plasmid production in selected E. coli strain
- Fermentation option up to 200 L
- Harvesting by centrifugation / Tangential Flow Filtration (TFF)
- Cell lysis
-
- Chromatographic purification
- Formulation by Tangential Flow Filtration (TFF)
-
- Formulation
- Fill and Finish
-
- QC release testing
- QP Certification and full GMP documentation
Plasmid Production Services – Flyer
Download a Plasmid flyer
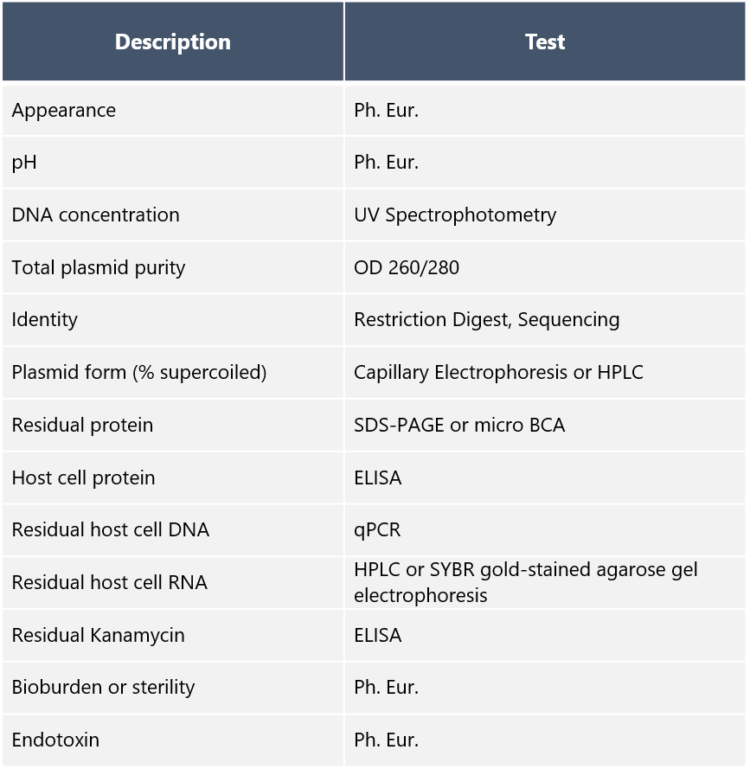
DownloadPlasmid QC Test Package Example
The QC tests for plasmids include e.g. the following:
Plasmid QA Documentation Package Example
Biovian has a strong track record in supporting clients with regulatory-compliant documentation. The documentation packages for Plasmid DNA projects include e.g. the following:
- TSE/BSE certificate
- Certificate of Analysis
- Certificate of GMP compliance
- Comprehensive production summary report
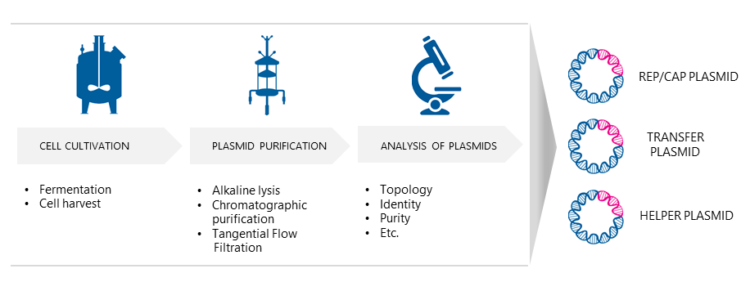
Production of AAV plasmids
Three separate E. coli processes are needed to produce the necessary AAV plasmids for transfection. The bacterial cells are cultured under optimized growth conditions. The microbial cells are then harvested using centrifugation. To release the intracellular plasmids the cultured host cells are disrupted using alkaline lysis. Then the AAV plasmids are purified with chromatographic methods. Finally, the plasmids will be analyzed e.g. for their identity and purity, and E.coli master and working cell banks are generated.