Track record in viral vector development and manufacturing
One-Stop-Shop CDMO services in viral vector production
Our viral vector services span from plasmid DNA manufacturing to Qualified Person-approved release of the final labeled Drug Product. We can provide access to some of the newest production platforms through our partnerships.
-
- Research Cell Bank
- Master Cell Bank and Working Cell Bank
- Master Viral Seed Stock and Working Seed Stock
-
- Suspension cell culture in single-use bioreactors – up to 200 L
- Adherent cell culture in multilayer flasks, packed-bed bioreactor and single-use bioreactors on microcarriers
-
- Ultracentrifugation-based Downstream Processes
- Chromatography-based Downstream Processes
- Comprehensive purification solutions – chromatography, membrane processes, Tangential Flow Filtration processes (TFF)
-
- Formulation and final Drug Product manufacturing
- Automated aseptic filling line for live viral vectors
- Typical batch sizes 200 – 1000 vials
-
- Product-specific assays
- Impurity analysi
- Cell-based assays
- Microbiological QC and safety assays (sterility, bioburden, endotoxin etc.)
- QP certification and full GMP documentation
Viral vector GMP production facility
The state-of-the-art production facility allows for flexible viral vector production scenarios. The cleanroom class C (EU GMP) and biosafety level 2 facility is designed for the manufacturing of GMP-grade viral vectors. The space design follows a modular ballroom concept and unidirectional process flow. The equipment are mobile, allowing for easy reconfiguring, and the operations are performed according to one-way process principles from incoming to outgoing material. Production is campaign-based – a single product is produced at a time, and disposable materials and closed systems are used. Open processes are executed in biosafety cabinets with HEPA 14 filters, with air exhaust directly out of the building.
Utilities
- The production area is equipped with HVAC technology with a single-pass ventilation design, without any recirculation of air.
- The facility includes a restricted passage with electronic access control and pressured air-locks for material and personnel entry and exit. The airlocks are composed of two parts (D- and C-grade) and are controlled with inter-locks. HEPA filters are placed across the operating area and air-locks.
- Other utilities include gas supplies (CO2, O2, N2) and compressed air outlets.
- The facility is equipped with 24/7 monitoring and an alarm system.
Viral Vector GMP production – Video
Viral vector process development services
Biovian provides comprehensive viral vector process development services that are fully integrated with process analytics. Our process development services support the Quality-By-Design (QBD) approach from the earliest possible stage in order to enable a straightforward transition to GMP production.

ADENOVIRUS VECTORS
Adenovirus vector GMP production services
Adenoviruses are attractive tools for gene therapy, including immuno-oncology, because of their well-defined biology and characteristics including the possibility for large gene inserts. Biovian has proven expertise in adenovirus vector process development, GMP production, and analytics.
Biovian supports adenovirus vector production in both adherent cell cultures and suspension cell cultures. Scalable technologies enable a straightforward transition to GMP production. Sourcing GMP-grade raw materials and consumables from qualified material providers are included in the services. The One-Stop-Shop concept is finalized with bio-safety level 2 class aseptic filling in a grade A/B production suite, followed by clinical labeling, GMP storage, certification of GMP compliance and Qualified Person (QP) approved release.
GMP Adenovirus production workflow – An example
-
Cell and virus bank manufacturing
-
Process development/Technical transfer
-
Consistency batch
-
Engineering batch
-
GMP batch
Adenovirus vector upstream processes
The adenovirus vector upstream processes comprise 1) mammalian cell culture, e.g. HEK293, A549 in adherent or suspension culture, 2) transduction with adenovirus vector, 3) cell harvest and lysis, and 4) analysis of harvest. The selection of the producer cell line and culture type can be made together with our experts.
Suspension cell culture
Adenovirus vector production in suspension cultures enables easy upscaling and reduces process costs. Producer cell lines such as HEK293 cells can be adapted to suspension culture and grow in serum-free media, which enhances the safety profile. For small-scale suspension cultures shake flasks with various capacities can be used. For large-scale suspension cultures, the bioreactor options at Biovian include single-use stirred-tank bioreactors, where the GMP volume is up to 200 L. Depending on the project perfusion, fed-batch or batch cultivation will be applied.
Adherent cell culture
Adenovirus vectors can be produced in adherent cell cultures using T-flasks or multilayer flasks such as hyperflasks or cell stacks. Alternatively, adherent cell cultures can be carried out in bioreactors, where the options at Biovian are packed-bed bioreactors or single-use bioreactors with microcarriers. Adherent cultures require tissue-culture-treated vessels and repeated passaging, but allow for easy visual inspection under the microscope.
Suspension and adherent cell culture platforms and harvesting options
Laboratory scale
Suspension cell culture:
- Shake flasks, capacity options 24 mL-5L
- Single-use stirred-tank bioreactor up to 10 L
- Wave bioreactor 25 L
- Chemical lysis and clarification using depth filtration and microbial reduction filtration
Adherent cell culture:
- Cell factories
- T-flasks
- Hyperflasks
- Cell stacks
- Single-use stirred-tank bioreactor up to 10 L
- Wave bioreactor 25 L
- Packed-bed bioreactor up to 4 m2
- Mechanic or chemical detaching/lysis of cells
GMP production scale
Suspension cell culture:
- Shake flasks, capacity options 24 mL-5L
- Single-use stirred-tank bioreactor up to 10 L
- Wave bioreactor up to 200 L
- Chemical lysis and clarification using depth filtration and microbial reduction filtration
Adherent cell culture:
- Cell factories
- T-flasks
- Hyperflasks
- Cell stacks
- Single-use stirred-tank bioreactor up to 200 L
- Wave bioreactor 25 L
- Packed-bed bioreactor up to 500 m2
- Mechanic or chemical detaching/lysis of cells
Adenovirus vector downstream processes – GMP
Biovian’s platform approach to downstream processing of adenovirus vectors comprise 1) tangential flow filtration, TFF, for removal of impurities and buffer exchange 2) a capture step, where adenovirus vectors are purified and concentrated 3) polishing chromatography 4) concentration and formulation with TFF, or dialysis for low volumes, and 5) analysis of the Drug Substance according to regulatory requirements. For small-scale production, fast gradient ultracentrifugation- based method can be used to purify the clarified adenovirus vector harvest. The final filtration is performed using a 0.2 µm filter to fulfill the sterility requirement for adenovirus DS.
-
Tangential flow filtration, TFF
-
Capture step
-
Polishing chromatography
-
Concentration and formulation
-
Analysis of drug substance
Adenovirus vector purification options
Ultracentrifugation-based purification
Chromatography purification
Laboratory scale:
- Chromatography through-put up to 150 mL/min
Production scale:
- Chromatography through-put up to 500 mL/min
TFF-based purification
Laboratory scale :
- Automated disposable TFF up to 200 mL/min
- Manual TFF system (small to midscale)
Production scale:
- Automated disposable TFF up to 18 L/min
Adenovirus vector Quality Control assays
Biovian offers full access to QC analytics for the demonstration of the quality of adenovirus vector GMP batches. We use compendial assays and perform sample-type specific validation to comply with all regulatory requirements for adenovirus vectors for human use. Development or technical transfer of product-specific analytical methods, as well as validation of these methods, is part of our services.
QC tests for adenovirus vector harvest
- Identification and genomic integrity: Immunochemical methods (Ph. Eur. 2.7.1), NAT (Ph. Eur. 2.6.21), or REA.
- Vector concentration: ddPCR or HPLC
- Infectious titer: ICC
- Extraneous agents: In vitro assays (Ph. Eur. 2.6.16)
- Sterility: Ph. Eur. 2.6.1
- Mycoplasma: Ph. Eur. 2.6.7
- Mycobacteria: Ph. Eur. 2.6.2
QC tests for control cells
- Identification: Identification and DNA fingerprinting (RAPD), Morphology (Ph. Eur. 5.2.3)
- Extraneous agents: In vitro assays (Ph. Eur. 2.6.16)
- Hemadsorbing viruses: Ph. Eur 2.6.16
QC tests for adenovirus vector Drug Substance and Drug Product
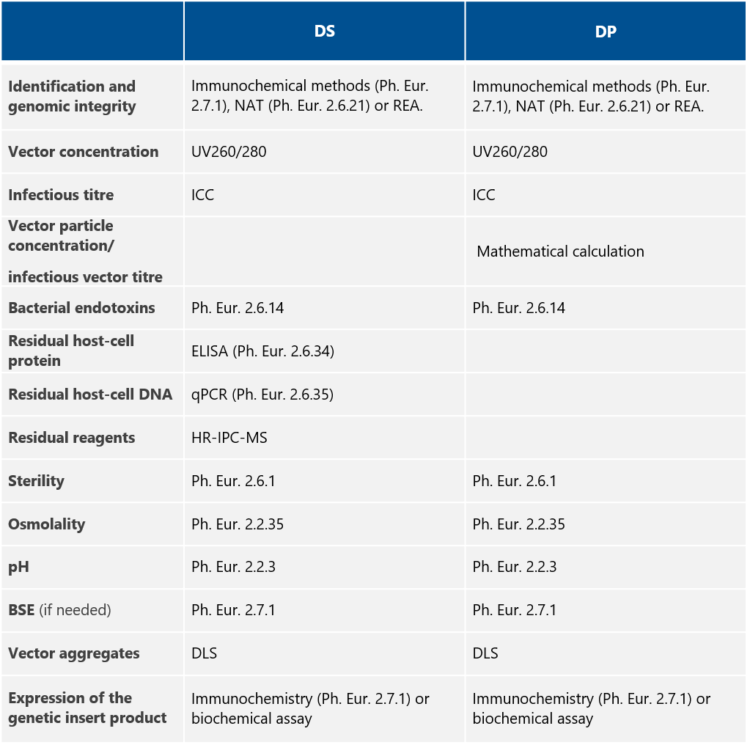
Adenovirus vector Fill and Finish
Aseptic Fill and Finish of adenovirus vectors is performed in dedicated suites that fulfill BSL 2 requirements. The automated filling line is validated for 2R and 10R vial sizes up to 1000 vials per session. The tubing, filling needle and other assemblies are fully disposable. In-house release testing services include sterility testing, and a 100% visual inspection is performed after each filling to ensure the safety of the adenovirus vector Drug Product in each vial. Before shipping the adenovirus vector products for use in clinical trials are certified by one of our Qualified Persons. We also provide intermediate GMP warehousing of the Drug Products at various temperatures (RT, +4°C, -20°C, and -80°C) on the same European Medicines Agency-certified site.
Adenovirus vector GMP documentation package:
- TSE/BSE certificate
- Certificate of analysis
- Batch certificate and certificate of GMP compliance
- GMP production summary report
CMC documentation
- IND/IMPD CMC documentation support upon request
Adenovirus vector stability studies
Stability testing of adenovirus vector Drug Substances and Drug Products is conducted at Biovian under controlled conditions according to ICH guidelines. The stability study services include study planning, analytical QC testing and reporting.

AAV VECTORS
AAV vector GMP production services
Adeno-associated virus (AAV) vectors are among the most frequently used viral vectors for gene delivery. Biovian’s support for AAV vector projects ranges from process development to clinical and commercial GMP production. We provide technologies for both adherent and suspension cell cultures that enable controlled upscaling.
Development or technical transfer of analytical methods is part of Biovian in-house services, as well as automated aseptic filling of viral vector products. Full GMP documentation and detailed batch records are provided, and our Qualified Persons are at your service to ensure that products are certified for clinical use before shipping.
GMP AAV vector production workflow – Example
-
Cell and virus bank manufacturing / Plasmid manufacturing
-
Process development / Technical transfer
-
Consistency batch / Up-scaling plan
-
Engineering batch
-
GMP batch
Production of AAV plasmids
AAV vector upstream processes
The AAV vector upstream processes comprise 1) mammalian cell culture, e.g. HEK293, or Sf9 insect cells 2) transfection with plasmids or transduction with baculovirus, depending on the selected approach and serotype 3) cell harvest and lysis, and 4) analysis of harvest. Biovian provides assistance with the selection of the producer cell line, as well as of culture type, based on the AAV serotype and other factors and needs. Access to some of the newest technologies and production platforms can be provided through our partnerships.
Suspension cell culture
Suspension cultures can be easily upscaled, as the limiting factor for cell growth is the concentration of cells in the medium. If needed, suspension cells can be cultured in serum-free media and are thus free from bovine-derived impurities such as TSE and BSE.
Adherent cell culture
Adherent cell culture is applicable for most cell types. Some AAV vector constructs only produce good yields in adherent cultures. Growth monitoring of adherent cell cultures can be done visually under the microscope. Adherent cell growth is limited by surface area, which may limit product yields.
Suspension and adherent cell culture platforms and harvesting options
Laboratory scale
Suspension cell culture:
- Shake flasks, capacity options 24 mL-5L
- Single-use stirred-tank bioreactor up to 10 L
- Wave bioreactor 25 L
- Chemical lysis and clarification using depth filtration and microbial reduction filtration
Adherent cell culture:
- Cell factories
- T-flasks
- Hyperflasks
- Cell stacks
- Single-use stirred-tank bioreactor up to 10 L
- Wave bioreactor 25 L
- Packed-bed bioreactor up to 4 m2
- Mechanic or chemical detaching/lysis of cells
GMP production scale
Suspension cell culture:
- Shake flasks, capacity options 24 mL-5L
- Single-use stirred-tank bioreactor up to 10 L
- Wave bioreactor up to 200 L
- Chemical lysis and clarification using depth filtration and microbial reduction filtration
Adherent cell culture:
- Cell factories
- T-flasks
- Hyperflasks
- Cell stacks
- Single-use stirred-tank bioreactor up to 200 L
- Wave bioreactor 25 L
- Packed-bed bioreactor up to 500 m2
- Mechanic or chemical detaching/lysis of cells
AAV vector downstream processes – GMP
The AAV vector downstream processes comprise 1) affinity chromatography for AAV capsid binding 2) anion exchange chromatography for removal of empty capsids and host cell contaminants 3) optional polishing step 4) exchange to formulation buffer using tangential flow filtration, and 4) analysis of Drug Substance. Biovian’s multi-step platform purification process is compatible with multiple AAV serotypes. For small-scale batches ultracentrifugation-based purification is available.
-
Affinity chromatography
-
Anion exchange chromatography
-
Polishing chromatography
-
Concentration and formulation
-
Analysis of drug substance
AAV vector purification options
Ultracentrifugation-based purification
Chromatography purification
Laboratory scale:
- Chromatography through-put up to 150 mL/min
Production scale:
- Chromatography through-put up to 500 mL/min
TFF-based purification
Laboratory scale :
- Automated disposable TFF up to 200 mL/min
- Manual TFF system (small to midscale)
Production scale:
- Automated disposable TFF up to 18 L/min
AAV vector quality control assays
Biovian provides full access to analytical methods that demonstrate the quality attributes mandatory for AAV vector GMP batches. Compendial assays and sample-type specific validation are performed to meet all regulatory requirements of AAV vectors for human use. Development or technical transfer of product-specific assays, including validation of assays, is part of our expert services.
QC tests for AAV vector harvest
- Identification and genomic integrity: Immunochemical methods (Ph. Eur. 2.7.1), NAT (Ph. Eur. 2.6.21), or REA.
- Vector concentration: qPCR, ddPCR
- Extraneous agents: In vitro assays (Ph. Eur. 2.6.16)
QC tests for control cells
- Identification: Morphology (Ph. Eur. 5.2.3), identification and DNA fingerprinting (RAPD)
- Extraneous agents: In vitro assays (Ph. Eur. 2.6.16)
- Hemadsorbing viruses: Ph. Eur 2.6.16
QC tests for plasmids
- Identification: Sequencing or NAT
- Genomic integrity: REA
- Concentration: OD260 or fluorescent dyes
- Residual host-cell DNA: qPCR
- Bacterial endotoxins: Ph. Eur. 2.6.14
- Sterility: Ph. Eur. 2.6.1
QC tests for purified AAV harvest or final bulk
- Identification: Immunochemical methods (Ph. Eur. 2.7.1), NAT (Ph. Eur. 2.6.21), or REA.
- Genetic characterization and genetic integrity: Sequencing (NGS, Sanger), PCR
- Vector concentration: qPCR, ddPCR
- Bacterial endotoxins: Ph. Eur. 2.6.14
- Residual host-cell proteins: ELISA (Ph. Eur. 2.6.34)
- Residual host-cell DNA: qPCR (Ph. Eur. 2.6.35)
- Residual reagents: With a suitable method
- Sterility: Ph. Eur. 2.6.1
Purified harvests may be pooled to a final bulk followed by sterility testing
(Ph. Eur. 5.14 Gene transfer medicinal product for human use, Sterility testing 2.6.1)
QC tests for AAV drug products
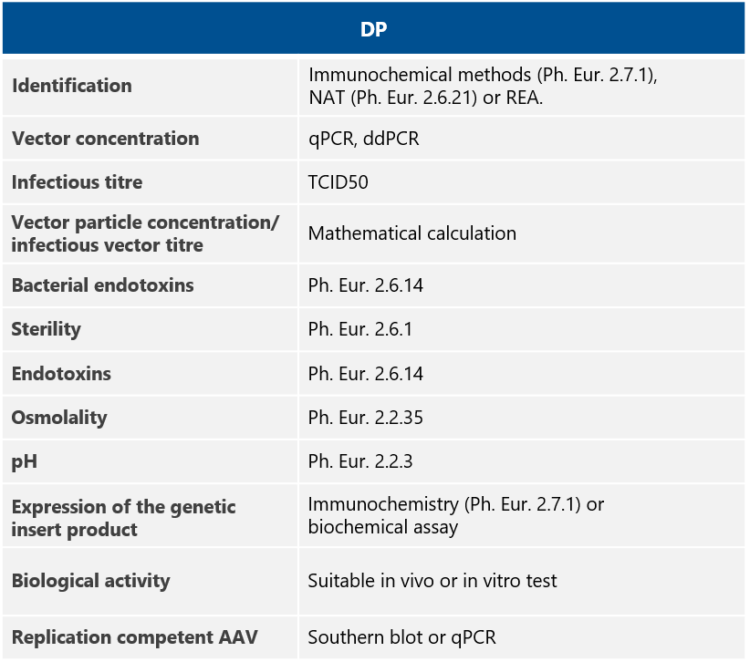
AAV vector Fill and Finish
Fill and Finish for AAV vectors is performed in a dedicated aseptic filling suite, grade A/B (EU GMP). The automated aseptic filling line for AAV vectors is validated for 2R and 10R vial-sizes up to 1000 vials per batch. The finished vials may either be kept in intermediate GMP storage at Biovian or be directly shipped to the clinic, once the batch has been released by one of our Qualified Persons.
AAV vector QA documentation package:
- TSE/BSE certificate
- Certificate of analysis
- Batch certificate and certificate of GMP compliance
- GMP production summary report
CMC documentation
- IND/IMPD CMC documentation support upon request
Stability testing
The stability testing services include the study plan, analytical QC testing, and reporting. Stability testing of AAV vector Drug Substances and Drug Products is conducted at Biovian according to ICH guidelines. We have controlled cabinets for stability studies according to ICH guidelines.


