Tenboron Ltd signs an agreement with Biovian for GMP contract manufacturing of a novel boron carrier for treating solid tumors
Tenboron Ltd, the developer of a novel boron carrier for use in Boron Neutron Capture Therapy (BNCT), has selected Biovian Oy as a CDMO partner for GMP manufacturing of their drug candidate for phase I clinical studies. The next generation boron carrier is designed to improve biochemically targeted radiation therapy of locally invasive malignant tumors, including head and neck cancers.
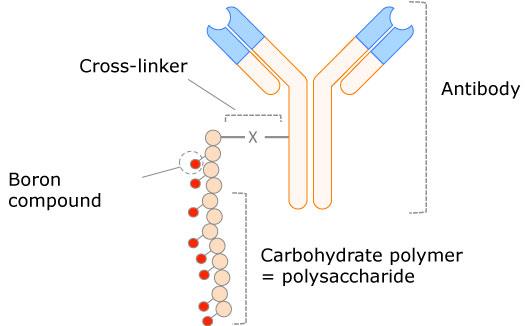
The agreement covers technical transfer, analytical method validation, and GMP manufacturing of boron carriers at Biovian’s facility in Turku, Finland. Aseptic filling and stability studies are also covered under the service agreement. Tenboron’s boron carrier has a high number of boron atoms (isotope-10B) that are chemically linked to an antibody targeting unit, which specifically directs the carrier to the cancerous cells. Thus, the boron-containing drug is administered directly into the cancerous tumor and is then activated by external neutron radiation. The short-range radiation leads to targeted cancer cell killing precisely at the site of boron accumulation.
Juha Jouhki, CEO of Tenboron Ltd:
We are very proud of getting closer to starting human trials with our novel boron carrier-based medicine. Selecting Biovian as a CDMO partner is an important step in reaching that milestone. I am confident that Biovian, which has extensive experience in biopharmaceutical manufacturing, will help us meet our project goals.
Knut Ringbom, CEO of Biovian:
We are very pleased that Tenboron has selected us as a partner for GMP manufacturing of their novel molecule. The service agreement that covers production, purification, filling and labeling is a good example of our One-Stop-Shop concept, where multiple services are provided under the same roof.


