Adenovirus vector GMP manufacturing in adherent cell cultures and suspension cell cultures.

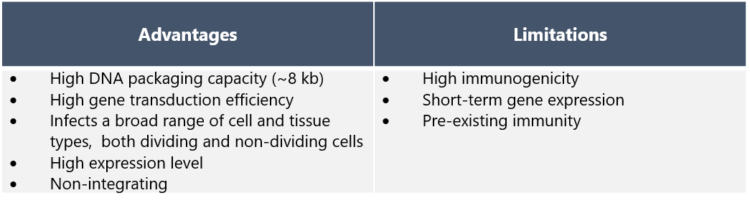
Adenoviruses are among the most attractive Viral Vectors for gene therapy because of their well-defined biology and characteristics. They have a high packaging capacity and can accommodate large transgene inserts of sizes up to ~8 kb. Adenovirus vectors also have high transduction efficiency and they can infect both dividing and non-dividing cells in a broad range of cell and tissue types. In contrast to e.g. lentiviruses, adenovirus DNA does not integrate into the host genome but remains episomal. Thus, adenovirus vectors are suitable for therapeutics that need a high, temporary gene expression, but they are not applicable for long term correction of monogenic disorders. Currently there are about 70 adenovirus vector-based products being developed or already on the market [1] Commercially available adenovirus vector-based products include Gendicine for treatment of head and neck squamous cell carcinoma, Zabdeno vaccine against Ebola and AZD1222 against COVID-19 [2,3,4].
In order to produce sufficient amounts of clinical-grade adenovirus vectors, GMP compliant cell banks and virus stocks need to be developed, and scalable production and purification methods need to be established. While there is no single production strategy that works for all adenovirus projects, Biovian provides the flexibility of multiple adenovirus production methods and the expert knowledge of different process steps. Biovian has developed processes and manufactured over 40 clinical adenovirus GMP batches for clients since 2004. Here Project Managers Johanna Silvola and Johanna Saari explain how adenovirus vector GMP production can be performed at Biovian in either adherent cell cultures or suspension cell cultures.
- Regulations for Adenovirus vector GMP manufacturing and testing
- Typical adenovirus vector project workflow
- Cell line selection
- GMP Cell Bank manufacturing
- GMP Virus Seed Stock manufacturing
- Adherent and suspension culture methods
- Harvesting of adherent and suspension cells
- Adenovirus vector purification and formulation
- Adenovirus vector Fill and Finish
Regulations for Adenovirus vector GMP manufacturing and testing

GMP manufacturing of adenovirus vectors for medicinal human use is a highly regulated process. Johanna Saari explains that the requirements demand that all starting materials, such as adenovirus seed stocks and cell banks, have to be manufactured and tested according to GMP requirements. “Also, all supporting material that is used in production has to be produced within a recognized quality system. The main regulatory guidance for adenovirus vector production is summarized in the table below:
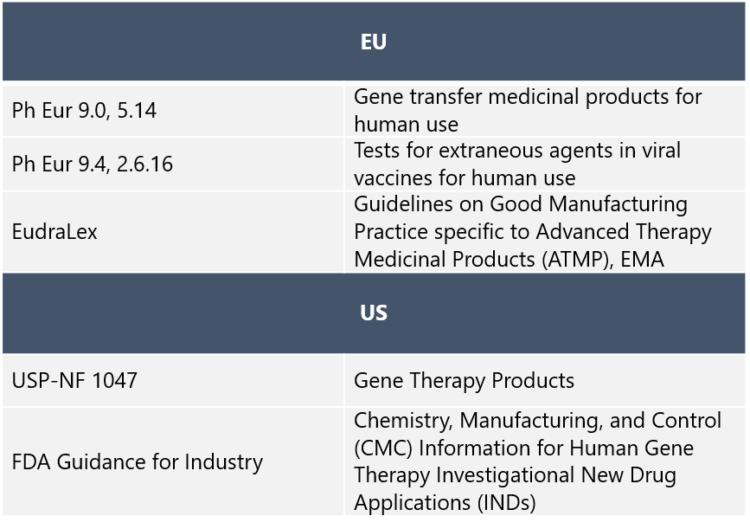
“An experienced CDMO can guide the client through the ATMP procedure, and source GMP-grade raw materials, components and reagents,” Saari says. “At Biovian we produce water for injections as well as GMP grade buffers in a dedicated production area. We routinely follow the requirements and standards provided by European regulatory authorities. US requirements can be implemented for the production and analytics if separately agreed,” Saari says. “Recently we also obtained the Accreditation Certificate of Foreign Drug Manufacturer, covering biological products from the Japanese Pharmaceuticals and Medical Devices Agency. This makes it possible for us to manufacture gene therapy products and other biologics to clients around the world.”
Typical adenovirus vector project workflow
Johanna Saari explains that a typical adenovirus vector project workflow consists of the following elements: 1) cell and virus bank manufacturing 2) process development or technical transfer 3) consistency batch 4) engineering batch and 5) GMP batch manufacturing.

Process development/technical transfer
To give a real-life example of an adenovirus vector process development project, Saari tells about a full development project for Ad5, involving both Upstream and Downstream Processes. “This particular project was aiming for the manufacturing of clinical trial material using a suspension culture. We developed a suspension culture process for a single-use stirred-tank bioreactor and performed a comparison against the Wave bioreactor. The culture media and infection parameters were optimized, as well as the actual cultivation process and the method of harvesting. We also developed and optimized the chromatography and TFF-based purification processes, including full in-process control sample analytics. And this is just one example – Biovian has been developing adenovirus vector processes since 2004 and our longest-lasting customer relationship has by now lasted more than10 years and is still active.”
Consistency batch
A consistency batch is a non-GMP, laboratory-scale batch, where valuable process data is gained before committing to full-scale manufacturing. Saari explains that the consistency batch manufacturing simulates the production scale processes in a scale-down process. “Scale-down models are used to gain knowledge about the process, which then can be used to optimize the operation conditions in a flexible environment before proceeding into the more restricted GMP environment. The aim is to confirm that the process itself, as well as the efficiency of the removal of product-related impurities, fulfills the pre-determined target values. It is also needed in order to confirm the product stability during the process and to determine product recovery,” she adds.
Engineering batch
An engineering batch is a non-GMP batch where the aim is to evaluate GMP conditions so that good batch records may be established. At Biovian the engeneering batch is produced in a GMP cleanroom using GMP production scale processes and GMP materials. What are the benefits of running an engineering batch? Saari replies that the purpose of the engineering batch is to confirm that the production scale processes fulfill the pre-defined process performance values, that the in-process control analytical results are within the targets and that the pre-determined end product specifications fulfill the acceptance criteria. “In short, an engineering batch is recommended because it helps to assure successful GMP campaign production. The engineering batch material may also be used to establish product shelf life by performing stability studies. Furthermore, engineering batch material may be used in preclinical toxicology studies and as quality control reference material.”
GMP batch
A successful engineering run is followed by the production of the GMP Drug Substance (DS) according to GMP procedures. “Our production is scheduled in a campaign-based flow in our dedicated GMO II, grade C, production suites. We use single-use disposable technologies and closed systems to eliminate the risk of cross-contamination,” Saari says. “Last year we more than doubled our capacity in Viral Vector manufacturing by opening a new state-of-the-art GMP facility.” Saari explains that each manufactured GMP Drug Substance batch is tested against the specifications, and when it has been shown to meet the acceptance criteria, formulation and aseptic Fill and Finish will follow. The Drug Product is then tested and released for clinical use by one of our qualified persons.
Cell line selection

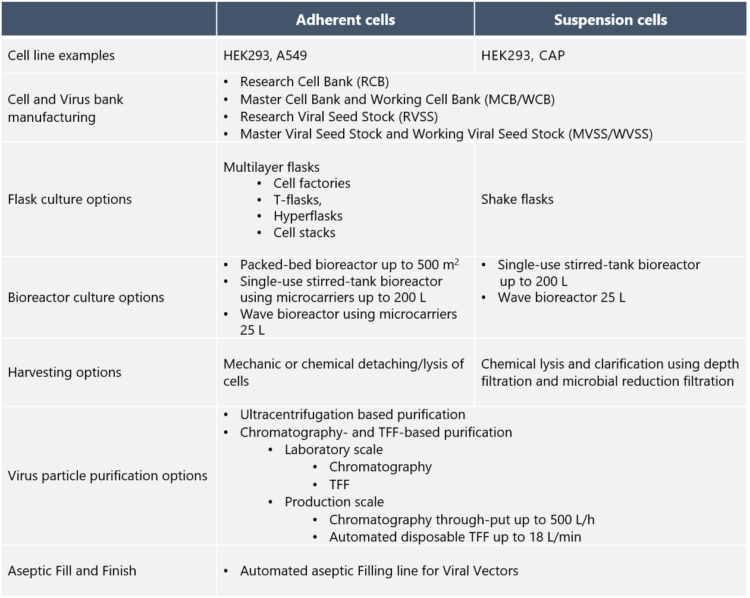
Typically, mammalian cell lines such as A549, HEK293 and CAP are used in the production of adenovirus vectors. Johanna Silvola explains that for safety reasons, adenovirus vectors are engineered to be replication-deficient, which means that their life cycle ends after infecting target cells in the patient. “We at Biovian assist with the selection of the producer cell line, based on the adenovirus culture type and other factors and needs”, she continues.
GMP Cell Bank manufacturing
After the cell line has been selected, cell banks are manufactured. Silvola explains that a Master Cell Bank (MCB) originates from a Research Cell Bank with a well-documented history. “A Working Cell Bank (WCB) is then prepared from a homogenous cell suspension obtained by culturing Master Cell Bank cells.”
GMP compliant Master and Working Cell Banks are manufactured in cleanroom areas where the environment is carefully monitored for viable and non-viable particulates. “Altogether Biovian has a 1300 m2 cleanroom footprint that comprises of 10 modular GMP suites”, Silvola says. “The processes of cell bank manufacturing are performed in cleanroom grade C suites, whereas open aseptic operations are carried out under biosafety grade A laminar flow hoods. Testing and characterization analysis of MCB and WCB is an essential part of the cell bank manufacturing. At Biovian we provide a full project management service for characterization of cell banks, conforming with major compendial and regulatory guidelines.”

GMP Virus Seed Stock manufacturing
“Biovian can start an adenovirus vector project from a single vial of virus or from a small volume of plasmids,” Silvola says. In the case where the client provides us with the virus seed, we proceed by manufacturing the Master Virus Seed Stock (MVSS) and the Working Virus Seed Stock (WVSS). The production protocol for the adenovirus seed stocks is typically similar to the protocol used in actual virus GMP manufacturing, but the batch size is smaller,” she says.
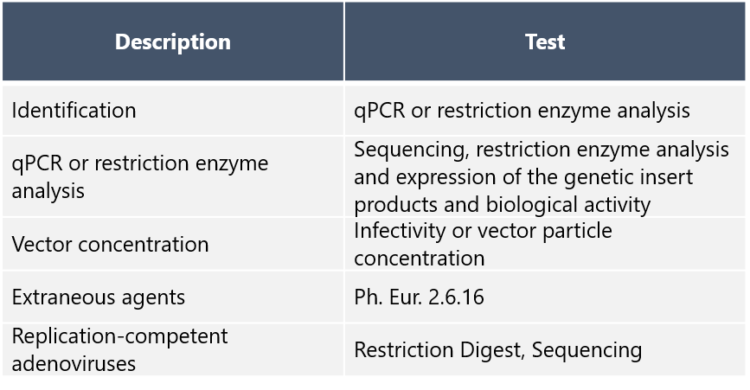
“Adenovirus seed stocks need to be well characterized and tested for identity, safety, purity, potency and sterility, just to mention a few parameters. These tests are mandatory as only a seed lot that complies with all requirements can be used for GMP production,” Silvola says. The required analyses for adenovirus seed stocks according to European Pharmacopoeia EP 5.14 are listed below:
Adherent and suspension culture methods
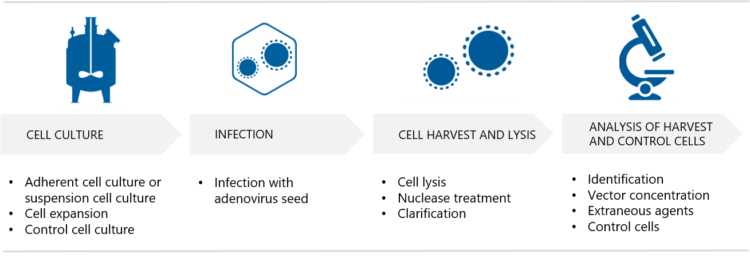
What are the culture options for the production cells? “If adenovirus vectors are produced in adherent cell cultures, one option is to use multilayer flasks such as T-flasks or hyperflasks”, Silvola replies. “Adherent cell cultures can also be carried out in bioreactors, where the options at Biovian include packed-bed bioreactors or single-use bioreactors with microcarriers. Adherent cultures require tissue-culture vessels and repeated passaging, but allow for easy visual inspection under the microscope,” she explains.
“Suspension cultures may sometimes be the preferred option for adenovirus production as they make scalability easier, reduce process costs and improve product quality,” Silvola says. “HEK293 cells can be adapted to suspension culture and can grow in serum-free media, which enhances the safety profile. On the other hand, suspension cultures require daily cell counts and viability determination for monitoring growth. A small-scale option for suspension cultures are shake flasks. For large-scale suspension cultures we provide bioreactor options such as single-use stirred-tank bioreactors, where the GMP volume is up to 200 L. Depending on the project we perform either perfusion, fed-batch or batch cultivation,” she says.
Harvesting of adherent and suspension cells
The method of harvesting the adenovirus-infected cells depends on the type of culture. Silvola explains that adherent cells are detached and lysed either mechanically or chemically. “Suspension cultures on the other hand are harvested chemically and clarified using depth filtration and microbial reduction filtration. The adenovirus vector harvest is then analyzed to demonstrate requirement compliancy. Analysis of the control cells is also part of this step,” she says. “Several harvests may be pooled before proceeding to the purification.”
Ultracentrifugation purification and formulation
According to Silvola, a fast, small scale purification option for the clarified and tested adenovirus vector harvest is gradient ultracentrifugation. “One way to do this is to use a two-step approach – the first step, gradient ultracentrifugation, generates a partially purified virus harvest and the second step, linear gradient ultracentrifugation, separates empty and full adenovirus particles. Then, the formulation is performed by using dialysis or tangential flow filtration technique. After adjusting the concentration, the final filtration is performed using a 0.2 µm filter to fulfil the sterility requirement for adenovirus DS,” she explains.
Chromatographic purification and formulation
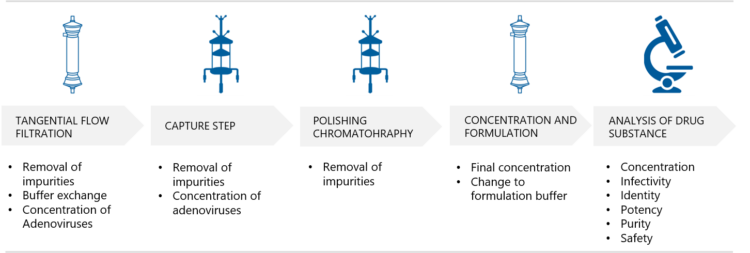
Silvola explains that Biovian uses a platform approach to the chromatographic purification of adenovirus vectors which includes both chromatographic and Tangential Flow Filtration (TFF) steps. “The purpose of the first TFF step is to remove impurities, such as proteins and to carry out buffer exchange. Thereafter the adenovirus vectors are purified and concentrated in the capture step. This is followed by polishing chromatography and a second TFF step for the final concentration and exchange to formulation buffer. With smaller quantities dialysis can also be applied. The final filtration of the adenovirus vectors is performed using a 0.2 µm filter. The purified adenovirus vector product is tested according to the regulatory requirements,” Silvola says.
Adenovirus vector purification and formulation
Adenovirus vector Fill and Finish
“To create the final Drug Product, Biovian provides Fill and Finish of adenovirus vectors using an automated aseptic filling line dedicated for BSL-2 class products,” Silvola says. “Our complete in-house Fill and Finish service naturally involves visual inspection, testing, labeling and packaging. The final step of the One-Stop-Shop services is the Qualified Person-approved release of the product for clinical trials. Furthermore, we take care of shipments according to the customer’s instructions. Intermediate warehousing of the Drug Products is also provided in our GMP storage, if that is what our client needs,” she concludes.
Summary
The manufacturing of adenovirus vectors for clinical use involves several parallel paths that need to comply with GMP regulations. In the above, Project Managers Johanna Saari and Johnna Silvola have described how GMP-grade adenovirus vectors are manufactured at Biovian in either adherent cell cultures or suspension cell cultures. Both emphasize that expertise in process development, up-scaling, analytics and GMP regulations is essential for the success of an adenovirus vector project, aiming for clinical trials.
References
[1] Global Data
[2] China approves first gene therapy, Nat Biotechnol. 2004; 22(1): 3–4 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7097065/
[3] Zabdeno https://www.ema.europa.eu/en/medicines/human/EPAR/zabdeno
[4] AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19 https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html


