Viral Vector and gene therapy basics summarized
Here we present some basic concepts of Viral Vectors used in gene therapy applications with special focus on AAV and Adenovirus vectors. Please proceed to this page if you are looking for information about our Viral Vector process development and manufacturing services.
In brief
Viral Vectors utilize the powerful ability of viruses to enter cells and thus deliver genetic material. Unlike natural viruses, Viral Vectors do not cause disease since they have been modified by removing e.g. the genes involved in replication. These are replaced with therapeutic genes. Viral Vectors are manufactured in packaging cell lines such as HEK293 or A547. Viral Vector-based gene therapy can be used for treating both inherited and acquired diseases. A few gene therapies have reached the market and they are used to treat patients with rare diseases or certain forms of cancer. Hundreds of additional therapies are currently under development.
An ideal Viral Vector
An ideal Viral Vector is genetically stable, safe to handle, non-toxic for host cells, has a high packaging capacity and transduction efficiency, and does not elicit an immune response. Several Viral Vector types have been used in gene therapy research and clinical studies during the past decades. Adeno-Associated Viral (AAV) vectors, Adenovirus vectors, Lentivirus vectors and Gammaretrovirus vectors are the four main groups being used. Each of them has its unique characteristics, which affect their use as vehicles in gene therapy.
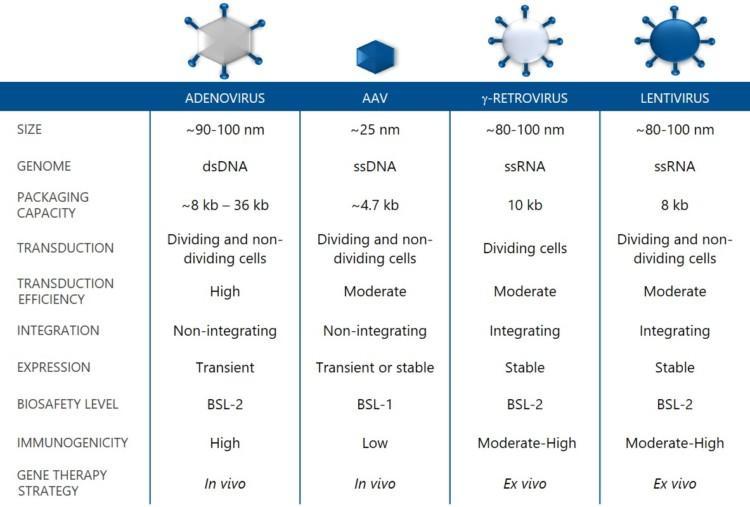
Integrating vs non-integrating vectors
Integrating Viral Vectors, such as lentivirus and gammaretrovirus vectors, fuse a fragment of their genetic material into the host cell genome. These vectors are often characterized by stable and long-term expression of the transgene. Genetic integration does not guarantee stable expression as transgene expression can be gradually silenced over time. Integrating Viral Vectors are preferred in applications where stable genetic modification needs to be maintained in dividing cells. CAR-T therapies such as Kymriah™ (Lentivirus vector) and Yescarta™ (Gammaretrovirus vectors) for certain types of blood cancers are commercially available applications, where integrating vectors are used ex vivo to modify T-cells [1,2]. The drawback with integrating Viral Vectors is safety-related, e.g. activation of oncogenesis in some applications [3].
In non-integrating Viral Vectors, such as AAV and Adenovirus vectors, the genetic material remains episomal in the cell cytoplasm. The main advantage is the low risk of genotoxicity caused by insertional mutagenesis [4]. Non-integrating Viral Vectors can provide stable transgene expression in non-dividing postmitotic cells, such as neurons, and transient or stable expression in dividing cells. If stable expression in dividing cells is required, repeated dosing of non-integrating vectors may be an option, as long as the immune response can be managed [5].
Which Viral Vector to select?
The suitability of a Viral Vector for a particular application depends on several factors, including packaging capacity, type of target cells and desired duration of gene expression. Immuno-oncology and vaccines are among the primary applications for Adenovirus vectors. Lenti- and Gammaretrovirus vectors are predominantly used for ex vivo gene therapy of transplantable stem cells. The AAV vectors are favoured as vehicles for direct in vivo gene delivery.
In vivo vs ex vivo gene therapy
- In vivo gene therapy – Viral Vectors carrying a transgene are directly administrated to the patient either by intravenous infusion (i.e. systemic administration) or by intraocular, intramuscular, intrathecal, or intraparenchymal injection into the organ of interest (i.e. local administration).
- Ex vivo gene therapy – the patient’s cells are extracted and genetically modified with Viral Vectors outside the body. The treated and cultured cells are then reintroduced into the patient by intravenous infusion or intramuscular or intrathecal injection.
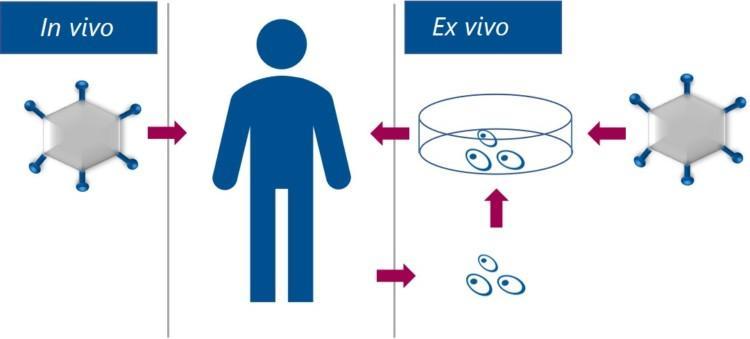
In vivo gene therapy and ex vivo gene therapy using Viral Vectors
AAV vectors
The wild type Adeno-Associated virus was discovered over 50 years ago, and it was first thought to be a contaminant of Adenovirus preparations [6]. As a dependoparvovirus, AAV depends upon Adenovirus co-infection for replication. Although AAV has a high prevalence in humans, it is non-pathogenic and prompts a very mild immune response. AAV consists of an icosahedral capsid, which is comprised of three viral proteins (VP1, VP2, VP3) and encapsulates a 5kb single-stranded DNA genome. About a dozen naturally occurring AAV serotypes can be differentiated based on surface antigen expression and amino acid sequence differences. The broad range of AAV serotypes is essential for Viral Vector applications because the serotype determines the tropism, i.e.–the ability of the vector to infect different cell types or tissues. Additional AAV vector serotypes can be created by capsid design [7]. In simple terms, new AAV vector-based treatments can be generated by replacing the capsid and the transgene.
Advantages of AAV vectors
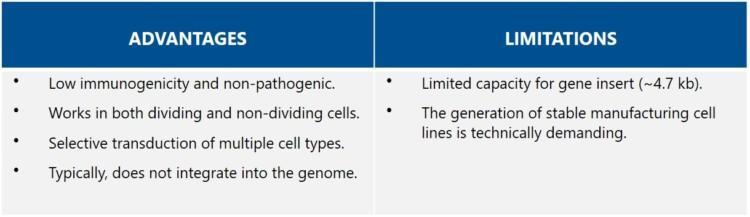
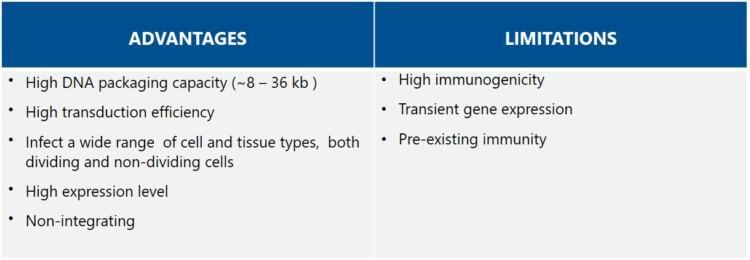
AAV vectors are considered attractive vectors for gene therapy because of their high safety profile. They are low in immunogenicity and do not integrate into the host genome (i.e., they remain episomal). The selection of AAV vector serotypes is rapidly expanding through de novo capsid design, allowing for targeting of specific tissues or cell types.If coupled with tissue-specific promoters, targeted expression is further improved [8].
Limitations of AAV vectors
The paramount limitation of AAV vectors is the small size of the therapeutic gene insert. The maximum length is about 4.7 kb, which makes targeting of larger genes and consequently, particular diseases, challenging. Strategies such as dual vectors or minigenes have been developed to overcome the size limitation [9]. Another constrain is that a large amount of AAV vectors is needed for efficient transduction, but there are inefficiencies in AAV vector production. A further challenge is the substantial amount of empty capsids in the crude product, which requires an additional purification step. There are also concerns about possible genotoxicity in case elements of the immortalized packaging cells get into the AAV vectors.
AAV vector production systems
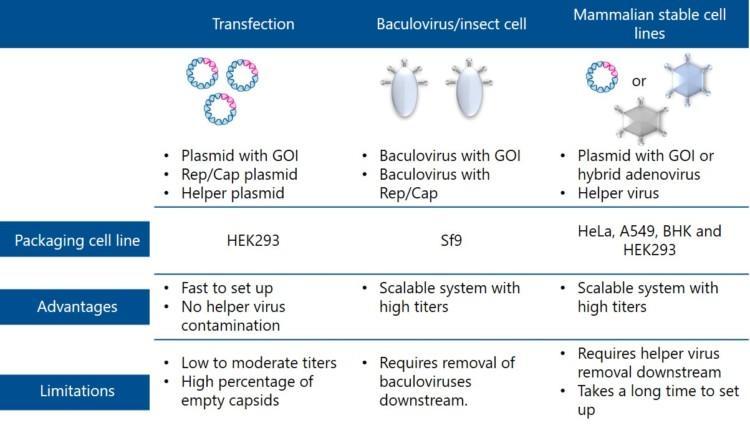
Three AAV vector production systems are described below. They differ in how the components needed for virus manufacturing are provided.
Transfection – using lipid-based methods the packaging cells, commonly HEK293, are transfected with three DNA plasmids harbouring all the AAV components. The first plasmid contains the genes needed for replication (Rep genes) and for the capsid proteins of the desired serotype (Cap genes). The second plasmid, called “helper plasmid”, carries the adenovirus genes (E2, E3, E4, and VARNA) required to support AAV replication. The third plasmid carries the therapeutic transgene. The Rep and Cap genes and the adenoviral helper genes may also be combined into one plasmid, which means that only two plasmids are needed for the transfection of the mammalian cells. Transfection is free from helper virus contaminants and allows for fast production of small batches. However, titers produced with transfection are low to moderate. The crude AAV product contains a high percentage of empty capsids or inactive particles. Another drawback is that a massive amount of expensive plasmids must be used as raw material, complicating scale-up from benchtop to GMP manufacturing.
Baculovirus/insect Sf9 cells – this production platform uses the capability of baculovirus to deliver AAV component genes into Sf9 producer cells. In a dual-baculovirus-Sf9 production system two recombinant baculovirus types are used sequentially to transduce cells. One baculovirus system carries genes for replication and capsid formation (Rep and Cap genes), and the other the therapeutic transgene. Also, stable Sf9 insect cell lines expressing Rep and Cap proteins have been developed to enable infection with only one recombinant baculovirus. The most significant advantage of this platform is its scalability, GMP compatibility, and 10-fold higher titers compared to transfection. Several serum-free media are available for Sf9 cell growth in suspension. However, baculovirus-related impurities may be a problem [10].
Stable cell lines – these cell lines either constitutively or upon induction express some AAV vector components, needed for replication and capsid expression (Rep and Cap genes). Infectious AAV vectors can be generated by transfecting the cells with plasmids containing the therapeutic transgene or transducing the cell line with hybrid viruses. Helper viruses such as a wild-type Adenovirus (Ad5) or herpes simplex virus (HSV-1) trigger the production of AAV vectors by providing additional genes. Stable cell lines, based on HeLa, CAP, A549, BHK, and HEK293 cells, have been developed for AAV vector production. Stable cell lines are well suited for production scale-up and they enable the generation of high titers of clinical-grade vectors. However, screening of stable and high-producer clones is time-consuming and limits the wide usage of these systems. Screening of thousands of clones may be required to obtain a high producer cell clone for a specific vector. These systems also produce helper viruses as a by-product (residual Adenovirus or residual HSV) that need to be effectively removed.
Examples of commercially available AAV vector-based treatments
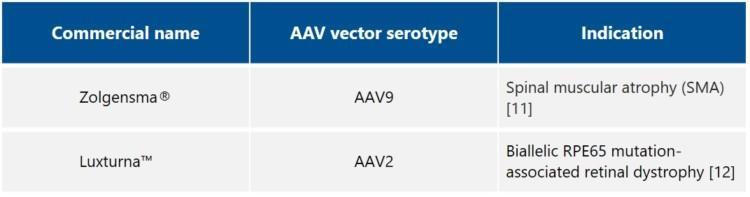
Examples of indications for which clinical trials are ongoing include Hemophilia A, Hemophilia B, Choroideremia, Retinitis pigmentosa, Fabry disease, Pompe disease, Hunter syndrome, Parkinson’s disease, Batten disease, Duchenne Muscular Dystrophy (DMD), Spinal Muscular Atrophy (SMA), and Beta-thalassemia [13, 14].
Adenovirus vectors
Adenoviruses were among the first viruses investigated as potential gene therapy vectors. Wild-type Adenoviruses are known to cause respiratory tract infections, .i.e., “the common cold”, in humans, and they can also infect other organs such as the brain and bladder. More than 50 distinct Adenoviral serotypes have been found that infect humans, and serotypes 2 and 5 are the most extensively characterized ones. There are also over 30 non-human primate Adenoviruses that have been isolated and characterized, e.g. from chimpanzees.
Adenovirus vectors in gene therapy
Adenovirus vectors are commonly engineered by removing the E1 region, responsible for replication, and other “early phase” genes, and replacing them with the transgene. The packaging capacity of ordinary Adenovirus vectors is ~8 kb. Adenovirus vectors are produced in HEK293 cell lines with stably express E1 proteins allowing for replication and packaging. Nowadays it is also possible to engineer Adenovirus vectors by removing almost all viral genes. These so-called “high-capacity”, “gutless”, or “helper-dependent” Adenovirus vectors can accommodate inserts as large as ~36 kb. They are attractive not only because of significantly larger capacity, but also because of stable expression and reduced immunogenicity [15,16]. Production of such Adenovirus vectors requires modified helper viruses and modified HEK293 producer cell lines. Helper virus contaminants need to be effectively removed from the final preparation, making the production of high-capacity Adenovirus vectors more challenging.
Advantages of Adenovirus vectors
Adenoviruses are attractive vehicles for gene therapy mainly because of their high packaging capacity. Adenovirus vectors can also transfect both dividing and non-dividing cells in a broad range of cell and tissue types. The Adenovirus DNA does not integrate into the host genome but remains episomal. Thus Adenovirus vectors are suitable for therapeutics that need high, but temporary, gene expression. Some vaccines and oncolytic therapies benefit from the immunogenicity and cellular toxicity of the Adenovirus vectors, but constitute a major obstacle for most gene therapies.
Limitations of Adenovirus vectors
The most significant limitation of Adenovirus vectors is pre-existing immunity in patients and the substantial immune response they may trigger. Most adults have been exposed to Adenoviruses, as they are common pathogens in humans, andthus the immune system will also attack the Adenovirus-based therapeutic vector. If this is the case, the body will recognize the proteins on the Adenovirus vector shells and destroy them before the therapeutic gene is delivered. Several strategies are being employed to circumvent pre-existing immunity against Adenovirus vectors, such as the use of rare human serotypes or non-human Adenovirus vectors, e.g., chimpanzee-derived, shielding the surface of Adenoviruses with polyethylene glycol, or encapsulating the vectors into alginate microspheres [17]. Despite the developments in Adenovirus vector engineering, they can still trigger host immunogenicity and cellular toxicity [18].
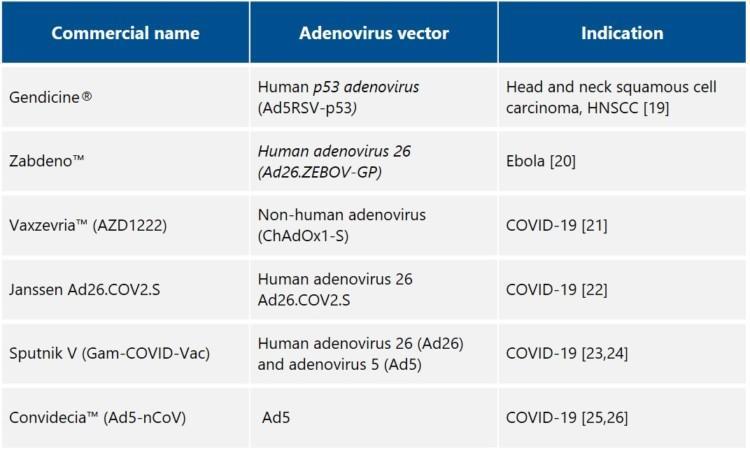
Examples of commercially available adenovirus vector-based products
References
[1] Kymriah https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah
[2] Yescarta https://www.ema.europa.eu/en/medicines/human/EPAR/yescarta
[3] Erika Check. Regulators split on gene therapy as patient shows signs of cancer. Nature 2002; 419: 545–546. https://www.nature.com/articles/419545a#rightslink
[4] David RM, Doherty AT. Viral Vectors: The Road to Reducing Genotoxicity. Toxicol Sci. 2017;155(2):315-325.
[5] Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17 :295-304. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3044498/
[6] Atchison, R. W., Casto, B. C. & Hammon, W. M. Adenovirus-associated defective virus particles. Science 149, 754–756 (1965). 124. Matsushita, T. et al.
[7] Vandenberghe, L., Wilson, J. & Gao, G. Tailoring the AAV vector capsid for gene therapy. Gene Ther 2009; 16: 311–319 https://www.nature.com/articles/gt2008170
[8] Claire Domenger, Dirk Grimm. Next-generation AAV vectors—do not judge a virus (only) by its cover. Human Molecular Genetics 2019; 28: R3–R14. https://academic.oup.com/hmg/article/28/R1/R3/5526799?login=true
[9] McClements ME, MacLaren RE. Adeno-associated Virus (AAV) Dual Vector Strategies for Gene Therapy Encoding Large Transgenes. Yale J Biol Med. 2017; 19;90(4):611-623. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5733846/
[10] European Medicines Agency (EMA). (2012). Assessment report: Glybera. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002145/WC500135476.pdf.
[11] Zolgensma https://www.fda.gov/media/126109/download
[12] Luxturna https://www.fda.gov/media/109906/download
[14] https://www.ema.europa.eu/en/medicines/human/referrals/zynteglo
[15] Liu J, Seol DW. Helper virus-free gutless adenovirus (HF-GLAd): a new platform for gene therapy. BMB Rep. 2020;53(11):565-575. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7704218/#ref7
[16] Ricobaraza A., et al. High-Capacity Adenoviral Vectors: Expanding the Scope of Gene Therapy. Int J Mol Sci. 2020; 21;21(10):36. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7279171/
[17] Vemula SV, Mittal SK. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther. 2010;10(10):1469-87https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2951029/
[18] Wang, Q. & Finer, M. H. Second-generation adenovirus vectors. Nat. Med. 2, 714–716 (1996).
[19] http://www.sibiono.com/en/productinfo.aspx
[20] Zabdeno https://www.ema.europa.eu/en/documents/overview/zabdeno-epar-medicine-overview_en.pdf
[21] Vaxzevria https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca
[22] Janssen Ad26.COV2.S https://www.fda.gov/media/146217/download
[23] Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00234-8/fulltext
[24] Sputnik V https://www.ema.europa.eu/en/news/ema-starts-rolling-review-sputnik-v-covid-19-vaccine
[25] http://www.cansinotech.com/html/1///179/180/651.html
[26] https://www.reuters.com/article/us-healthcoronavirus-china-vaccine-idUSKBN2AO0GH


